

Description
Modification of glass, Si, and PDMS with this silane opens exciting possibilities for surface immobilization of acetylene-bearing molecules through the so-called Sharpless "click" chemistry (Angew. Chem. Int. Ed. 2002, 41, 2596-2599). In this reaction, the azide groups on the monolayer react with acetylene groups of a molecule of interest to form a stable triazole linkage. The reaction is quantitative and regioselective, yields a single product at a single orientation, and is orthogonal to most other coupling schemes.
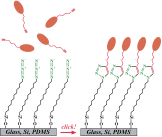
Recently (J. Am. Chem. Soc. 2005, 127, 8600-8601), scientists from Stanford University have shown that it is ideally suited for immobilization of biomolecules (e.g., oligonucleotides). We collaborated with the Stanford team to commercialize this exciting product in a record-breaking time? We hope it will prove useful in your experiments!
Note: Please see the thiol versions of this compound (Cat. # FT 010 under Functionalized Thiols and TH 008 under Thiols for Bioresistant and Biospecific SAMs). Using these compounds, chemical ligation can be coupled with electrocemistry to allow for immobilization of different types of molecules onto different locations of the substrate (J. Am. Chem. Soc. 2006; 128, 1794-1795).
Standard Products
| name | catalog no. | m | n | weight | price |
|---|---|---|---|---|---|
| MeO3SiC11-N3 | SI 005-m11-0.2 | 11 | 200 mg | Log in to see the prices | |
| MeO3SiC11-N3 | SI 005-m11-0.5 | 11 | 500 mg | ||
| MeO3SiC11-N3 | SI 005-m11-1 | 11 | 1 g |
Custom synthesis variants
This product is also available on custom synthesis basis, in the following variants:
m: 8, 10, 17
Note, if none of the listed structures fit your specific requirements, please submit your own structure formula within our custom synthesis contact form by clicking button below.